Stop smoking

Stop smoking support
Learning to help your customers on their quit journey
10 min module

Top tips to help stop smoking
Additional advice to aid you in supporting customers to stop smoking
Printable PDF
Cough & Cold

Clearing a blocked nose
This short mode of action video shows how the new and unique formulation of Sudafed Plus Blocked Nose Spray (Xylometazoline, dexpanthenol) has a triple action to help clear a blocked nose
1 min video

Cough product range guide
A comprehensive guide to the Benylin range (diphenhydramine, levomenthol), with key product features and recommendation decision tree
Children’s health

Your guide to common childhood illnesses
This interactive video will test and reinforce your knowledge so you can confidently advise parents and carers on common childhood conditions
10 min video
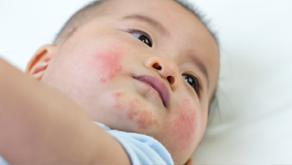
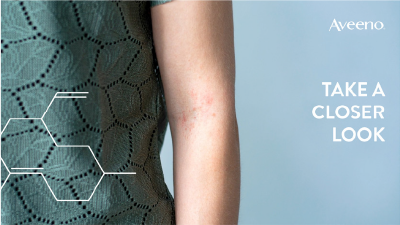
Advising parents on childhood eczema
Support parents and carers to manage their children’s dry skin
10 min module
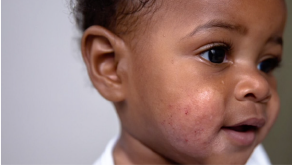
Optimising moisturiser use in childhood eczema
Update your knowledge on childhood eczema and how effective moisturiser use can help manage it
4 min video
Digestive health

Tackling myths about diarrhoea
Bust the myths surrounding diarrhoea treatment so you can help customers to recover
3 min video

Acute diarrhoea:
effective symptom management
Refresh your knowledge on treatment options and self-care advice to help ease symptoms of this common condition
10 min moduleAllergy
Quick access
Vaping Cessation
Stop smoking
Cough & cold
Children's health
- Your guide to common childhood illnesses
- Range guide
- Advising parents on childhood eczema
- Optimising moisturiser use in childhood eczema
Digestive health
Allergy
Calpol product information
Calpol Infant Suspension, Calpol Sugar-Free Infant Suspension (incl. Sachets) (Paracetamol)
Presentation:
Suspension containing 120mg Paracetamol per 5ml
Indication:
Treatment of mild to moderate pain and as an antipyretic. Can be used in many conditions including headache, toothache, earache, teething, sore throat, colds and influenza, aches and pains and post immunisation fever.
Dosage and Administration:
For the relief of fever after vaccinations at 2,3 and 4 months; 2.5ml up to 4 times a day starting at the time of vaccination. If your baby still needs this medicine two days after receiving the vaccine talk to your doctor or pharmacist. Dosage for the relief of pain and other causes of fever in babies aged 2-3 months (if your baby weighs over 4kg and was born after 37 weeks); 2.5ml. If necessary give a second dose after 4-6 hours. Dosage for Children over 3 months; Children 3 to 6 months: 2.5 ml. Children 6 to 24 months: 5 ml. Children 2 years to 4 years: 7.5 ml. Children 4 to 6 years: 10ml. Do not give more than 4 doses in 24 hours and leave at least 4 hours between doses.
Contraindications:
Hypersensitivity to paracetamol or other ingredients.
Precautions:
Do not exceed recommended dose or take with other paracetamol-containing medicines. Caution in severe hepatic or renal impairment and chronic alcohol use. Interactions with domperidone, metoclopramide, cholestyramine, anticoagulants, alcohol, drugs that induce hepatic microsomal enzymes, carbamazepine, fosphenytoin, phenytoin, phenobarbital, and primidone (also isolated reports of hepatotoxicity). Due to the presence of sucrose and sorbitol in the Infant Suspension, patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrose-isomaltase insufficiency should not take this medicine. Due to the presence of maltitol liquid and sorbitol liquid in the Sugar Free Suspension, patients with rare hereditary problems of fructose intolerance should not take this medicine. Sorbitol and maltitol (Sugar-Free only) may have a mild laxative effect. Parahydroxybenzoates and carmoisine may cause allergic reactions. Benzyl alcohol (Sugar-Free only) may cause allergic reactions. Large amounts of benzyl alcohol can build-up in your body and may cause metabolic acidosis. Patients should be informed about the signs of serious skin reactions and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Fertility, pregnancy and lactation:
Consult doctor before use.
Side effects:
Uncommon: nephropathy toxic.
Very rare: hypersensitivity, anaphylactic reactions and rash.
Not Known: blood disorder (including thrombocytopenia and agranulocytosis), liver injury, fixed eruption, rash pruritic, urticaria, renal papillary necrosis, and transaminases increased.
Very rare cases of skin reactions. Chronic hepatic necrosis has been reported.
RRP (ex VAT): 60ml bottle (sugar free only): £2.49; 100ml bottle: £3.58; 200ml bottle: £5.91; 12 x 5ml sachets (sugar free only): £3.74; 20 x 5ml sachets (sugar free only): £6.08
Legal category: 200ml bottle: P; 100ml bottle: GSL; 60ml bottle (sugar free only): GSL; Sachets (Sugar-free only): GSL
PL holder: McNeil Products Ltd, 50-100 Holmers Farm Way, High Wycombe, Buckinghamshire, HP12 4EG
PL number: Calpol Infant suspension: 100ml bottle: 15513/0122; 200ml bottle: 15513/0004; Calpol Sugar-free Infant Suspension: 60ml and 100ml bottle: 15513/0123; 200ml bottle: 15513/0006; Sachets:15513/0155
Date of preparation: 06 May 22
Nicorette product information
Nicorette QuickMist 1mg/spray mouthspray (nicotine), Nicorette QuickMist Cool Berry 1mg/spray mouthspray (nicotine) & Nicorette Quickmist SmartTrack 1mg/spray Mouthspray (nicotine)
See SmPC for full information
Presentation:
Oromucosal spray. Each 0.07 ml contains 1mg nicotine, corresponding to 1mg nicotine/spray dose.
Uses:
Relieves and/or prevents craving and nicotine withdrawal symptoms associated with tobacco dependence. It is indicated to aid smokers wishing to quit or
reduce prior to quitting, to assist smokers who are unwilling or unable to smoke, and as a safer alternative to smoking for smokers and those around them. It is
indicated in pregnant and lactating women making a quit attempt.
Dosage:
Adults and Children over 12 years of age: The patient should make every effort to stop smoking completely during treatment with Nicorette QuickMist. One or two sprays to be used when cigarettes normally would have been smoked or if cravings emerge. If after the first spray cravings are not controlled within a few
minutes, a second spray should be used. If 2 sprays are required, future doses may be delivered as 2 consecutive sprays. Most smokers will require 1-2 sprays
every 30 minutes to 1 hour. Up to 4 sprays per hour may be used; not exceeding 2 sprays per dosing episode and 64 sprays in any 24-hour period. Nicorette
QuickMist should be used whenever the urge to smoke is felt or to prevent cravings in situations where these are likely to occur. Smokers willing or able to stop
smoking immediately should initially replace all their cigarettes with the Nicorette QuickMist and as soon as they are able, reduce the number of sprays used
until they have stopped completely. When making a quit attempt behavioural therapy, advice and support will normally improve the success rate. Smokers
aiming to reduce cigarettes should use the mouthspray, as needed, between smoking episodes to prolong smoke-free intervals and with the intention to reduce
smoking as much as possible.
Contraindications:
Children under 12 years of age. Known hypersensitivity to nicotine or any excipients in the mouthspray.
Precautions:
Underlying cardiovascular disease, diabetes mellitus, G.I disease, uncontrolled hyperthyroidism, phaeochromocytoma, hepatic or renal impairment, seizures. Stopping smoking may alter the metabolism of certain drugs. Transferred dependence is rare and both less harmful and easier to break than smoking
dependence. May enhance the haemodynamic effects of, and pain response to, adenosine. Due to the presence of a small amount of butylated hydroxytoluene
(BHT), this medicine may cause local skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes. Keep out of reach and sight of
children and dispose of with care. Care should be taken not to spray the eyes whilst administering the spray.
Pregnancy & lactation:
Smoking cessation during pregnancy should be achieved without NRT. However, if the mother cannot (or is considered unlikely to) quit without pharmacological support, NRT may be used only after consulting a healthcare professional.
Side effects:
Very common: Headache, throat irritation, nausea, hiccups.
Common: Toothache, cough, hypersensitivity, burning sensation, dizziness, dysgeusia, paraesthesia, abdominal pain, diarrhoea, dry mouth, flatulence, salivary hypersecretion, stomatitis, vomiting, dyspepsia, fatigue.
Uncommon: Abnormal dreams, palpitations, tachycardia, flushing, hypertension, bronchospasm, dysphonia, dyspnoea, nasal congestion, sneezing, throat tightness, eructation, glossitis, oral mucosal blistering and exfoliation, paraesthesia oral, dry skin, urticaria, angioedema, hyperhidrosis, pruritus, rash, erythema, pain in jaw, asthenia, chest discomfort and pain, malaise, oropharyngeal pain, rhinorrhea, gingivitis, musculoskeletal pain, hyperhidrosis.
Rare: Dysphagia, hypoaesthesia oral, retching.
Not known: Atrial fibrillation, anaphylactic reaction, blurred vision, lacrimation increased, dry throat, GI discomfort, lip pain, muscle tightness, angioedema, erythema, seizures.
RRP (ex VAT): Nicorette QuickMist 1mg/spray mouthspray: 1 dispenser pack £21.23, 2 dispenser pack £33.61, Nicorette QuickMist Cool Berry 1mg/spray mouthspray: 1 dispenser pack £21.23, 2 dispenser pack £33.61, Nicorette Quickmist SmartTrack 1mg/spray mouthspray: 1 pack dispenser £22.41, 2 dispenser pack £36.24
Legal category: GSL
PL holder: McNeil Products Ltd, 50-100 Holmers Farm Way, High Wycombe, HP12 4EG
PL number: Nicorette QuickMist 1mg/spray mouthspray: 15513/0357, Nicorette QuickMist Cool Berry 1mg/spray mouthspray: 15513/0395, Nicorette Quickmist SmartTrack 1mg/spray mouthspray: 15513/0357
Date of preparation: 13 June 22
Imodium product information
IMODIUM® Instants (loperamide)
Presentation:
White/off-white, circular, orodispersible tablet containing loperamide hydrochloride 2mg. Excipients with known effect: Each tablet contains 0.750 mg of Aspartame (E951) which is equivalent to 0.055 mg/mg and it contains less than 0.00066mg of benzyl alcohol. The Mint flavouring contains traces of Sulphites.
Uses:
Symptomatic treatment of acute diarrhoea in adults and children aged 12 years and over. Symptomatic treatment of acute episodes of diarrhoea associated with Irritable Bowel Syndrome in adults aged 18 years and over following initial diagnosis by a doctor.
Dosage:
Acute Diarrhoea: Adults and children over 12 years old: Two tablets initially followed by 1 tablet after every loose stool. Total daily dose should not exceed 6 tablets. Symptomatic treatment of acute episodes of diarrhoea associated with Irritable Bowel Syndrome in adults aged 18 years and over: Two tablets to be taken initially, followed by 1 tablet after every loose stool, or as previously advised by your doctor. Total daily dose should not exceed 6 tablets. Method of administration: Allow the tablet to disintegrate on the tongue and swallow; no liquid is needed.
Contraindications:
Hypersensitivity to loperamide or any excipient. Children under 12 years of age. Acute dysentery, characterised by blood in stools and high fever. Acute ulcerative colitis. Bacterial enterocolitis caused by invasive organisms. Pseudomembranous colitis associated with broad spectrum antibiotics. Conditions when inhibition of peristalsis is to be avoided due to the possible risk of ileus, megacolon or toxic megacolon. Discontinue promptly when ileus, constipation or abdominal distension develop.
Precautions:
Treatment with IMODIUM® Instants is symptomatic; give specific treatment when appropriate. The priority in acute diarrhoea is the prevention or reversal of fluid and electrolyte depletion, particularly in young children and in frail and elderly patients. Use of IMODIUM® Instants does not preclude the administration of appropriate fluid and electrolyte replacement therapy. Since persistent diarrhoea can be an indicator of potentially more serious conditions, IMODIUM® Instants should not be used for prolonged periods until the underlying cause of the diarrhoea has been investigated. If symptoms persist for more than 48 hours, consult a doctor. Patients with AIDS should stop therapy with IMODIUM® Instant Melts if abdominal distension develops. Contains benzyl alcohol, which may cause allergic reactions- Use with caution in hepatic or renal impairment, or in patients who are pregnant or breast feeding, because of the risk of accumulation and toxicity (metabolic acidosis). If IMODIUM® Instants are being used to control episodes of diarrhoea associated with Irritable Bowel Syndrome, consult a doctor if clinical improvement is not seen within 48 hours, for any changes in the pattern of symptoms or if there is a need for continuous treatment of more than 2 weeks. Patients should consult a doctor if aged 40 or over where it is some time since their last IBS attack or if symptoms differ to previous episodes; in casesof severe constipation; weight loss or loss of appetite; pain passing urine; blood is present in stools; episode after recent travel abroad. Some cases with extremely high doses had a fatal outcome. Cardiac events have been reported in association with overdose. Some cases with extremely high doses had a fatal outcome. Loss of consciousness, depressed level of consciousness, tiredness, dizziness, or drowsiness may occur when diarrhoea is treated with loperamide. Therefore, it is advisable to use caution when driving a car or operating machinery. Cardiac events including QT interval and QRS complex prolongation and torsades de pointes have been reported in association with overdose. Overdose can unmask existing Brugada syndrome. Patients should not exceed the recommended dose and/or the recommended duration of treatment.
Pregnancy & lactation:
Not recommended.
Side effects:
Common: headache, constipation, nausea and flatulence.
Uncommon: dizziness, somnolence, abdominal pain, abdominal discomfort, dry mouth, abdominal pain upper, vomiting, dyspepsia and rash.
Rare: Hypersensitivity reaction, anaphylactic reaction (including anaphylactic shock), anaphylactoid reaction, loss of consciousness, stupor, depressed level of consciousness, hypertonia, coordination abnormality, miosis, ileus (including paralytic ileus), megacolon (including toxic megacolon), abdominal distension, bullous eruption (including Stevens-Johnson syndrome, toxic epidermal necrolysis and erythema multiforme), angioedema; urticaria; pruritis; urinary retention; fatigue.
Not known: Acute pancreatitis.
RRP (ex VAT): 6 tablets £4.49; 12 tablets £6.87
Legal category: GSL
PL holder: McNeil Products Ltd, 50-100 Holmers Farm Way, High Wycombe, Buckinghamshire, HP12 4EG
PL number: 15513/0345
Date of preparation: 19 May 22
Benadryl product information
Benadryl Allergy Relief (Acrivastine 8mg)
Presentation:
Acrivastine 8 mg capsules.
Uses:
Symptomatic relief of allergic rhinitis. Also chronic idiopathic urticaria.
Dosage:
Adults and children aged 12 – 65 years: One capsule up to 3 times a day.
Contraindications:
Hypersensitivity to acrivastine or triprolidine or any excipients listed in section 6.1 of the SPC. Severe renal impairment. Rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption.
Precautions:
Concomitant administration of acrivastine with CNS depressants may produce additional impairment in mental alertness in some individuals. Patients with renal impairment should consult with a physician before use. This product may cause drowsiness. Acrivastine may cause dizziness and somnolence- caution when engaging in activities which require mental alertness until familiar with response to drug. Caution when taking with ketoconazole, erythromycin or grapefruit juice.
Pregnancy & lactation:
Not recommended.
Side effects:
Very common: somnolence.
Common:dry mouth, dizziness
Unknown: hypersensitivity (including dyspnoea and face swelling), rash.
RRP (ex VAT): 12s £4.58, 24s £7.78, 48s: £14.16
Legal category: 12s GSL, 24s GSL, 48s P
PL holder: McNeil Products Ltd, 50 – 100 Holmers Farm Way, High Wycombe, Buckinghamshire, HP12 4EG, UK
PL number: 12s & 24s 15513/0128, 48s 15513/0035
Date of prep: 07/12/2020
Sudafed
Sudafed Plus Blocked Nose 1mg/50mg/ml Nasal Spray Solution Product Information
Presentation:
Spray pump bottle containing 10ml of a clear, colourless to slightly yellowish solution. Each 1 ml of nasal spray contains 1 mg xylometazoline hydrochloride and 50 mg dexpanthenol.
Uses:
Symptomatic relief of nasal congestion associated with the common cold, influenza, sinusitis allergic and non-allergic rhinitis (vasomotor rhinitis), other upper respiratory tract allergies.
Adults and children 12 years and over:
One spray into each nostril up to 3 times a day, Maximum daily dose: 3 sprays in 24 hours. Use for more than 7 consecutive days is not recommended. Children under 12 years: Do not give to children under 12 years of age.
Contraindications:
Contraindicated in children under 12 years of age. Also contraindicated in individuals with the following conditions: hypersensitivity to the active substances or to any of the excipients listed, dry inflammation of the nasal mucosa (rhinitis sicca), taking or have taken monoamine oxidase inhibitors within the preceding two weeks, and with a history of transsphenoidal hypophysectomy or other surgical interventions which expose the dura mater.
Precautions:
Use with caution due to minimal systemic absorption with topically applied imidazoline sympathomimetics such as xylometazoline. Use of this product is recommended only after a careful assessment of the risks and benefits for cases of increased intraocular pressure, especially narrow‐angle glaucoma, serious heart and circulatory diseases (e.g. coronary heart disease, hypertension), phaeochromocytoma, metabolic disorders (e.g., hyperthyroidism, diabetes), porphyria, and prostate hyperplasia. Patients with long QT syndrome treated with xylometazoline may be at increased risk of serious ventricular arrhythmias. Use during chronic rhinitis may only be carried out under medical supervision. Prolonged treatment may lead to reactive hyperaemia of the nasal mucosa. Direct contact of the medicinal product with the eyes should be avoided.
Interactions:
Concomitant use with other sympathomimetics, antihypertensive agents, and medicines which potentially increase blood pressure should be avoided.
Pregnancy and Lactation:
Not recommended REF-PI-SU-0048
Side effects:
Uncommon: Hypersensitivity reaction (angioedema, skin rash, pruritus),
Rare:palpitations, tachycardia, hypertension.
Very rare: restlessness, insomnia, hallucinations, fatigue (drowsiness, sedation), headache, convulsions, arrhythmias, rebound congestion, nosebleed
Not Known:Sneezing, burning and dryness of the nasal mucosa.
Reporting of side-effects:You can report side-effects directly via the Yellow Card Scheme at Yellow Card | Making medicines and medical devices safer
RRP (ex VAT):£6.99
Legal category:P
PL holder:McNeil Products Ltd., 50 - 100 Holmers Farm Way High Wycombe, Buckinghamshire, HP12 4EG, UK
PL number:PL 15513/0407
Date of prep:03 AUG 2022
Benylin
Benylin Herbal Chesty Coughs Sugar Free Syrup(Ivy leaf, Sorbitol) Product Information
Presentation:
Brown, opalescent liquid containing 33 mg of extract (as dry extract) of Ivy leaf (Hedera helix L.) (DER 4-8:1). Extraction solvent ethanol 30% m/m and 2832 mg of Sorbitolper 4ml.
Uses:
Used to relieve chesty coughs associated with the common cold, based on traditional use only.
Adults, the elderly, and children aged 12 years and over:
The recommended dose is 4 ml of syrup two to three times daily using the graduated measuring spoon provided.
Contraindications:
Hypersensitivity to the active substance or to any of the excipients.
Precautions:
Do not exceed the stated dose. The use of this product in children under 12 years of age is not recommended because data are not sufficient and medical advice should be sought. If dyspnoea, fever or purulent sputum occurs, a doctor should be consulted. If the symptoms worsen, or persist longer than one week during the use of Benylin Herbal Chesty Coughs Sugar Free Syrup, a doctor or qualified healthcare practitioner should be consulted. Concomitant use with antitussives such as codeine or dextromethorphan is not recommended without medical advice. Caution is recommended in patients with gastritis or gastric ulcer. Benylin Herbal Chesty Coughs Sugar Free Syrup contains sorbitol.The additive effect of concomitantly administered products containing sorbitol (or fructose) and dietary intake of sorbitol (or fructose) should be taken into account. The content of sorbitolin medicinal products for oral use may affect the bioavailability of other medicinal products for oral use administered concomitantly. Each 4 ml of syrup contains 2832 mg of sorbitol (E 420). Patients with hereditary fructose intolerance (HFI) should not take/be given this medicinal product. Sorbitol may cause gastrointestinal discomfort and mild laxative effect.
Interactions:
Concomitant use with other sympathomimetics, antihypertensive agents, and medicines which potentially increase blood pressure should be avoided.
Pregnancy and Lactation:
Safety during pregnancy and lactation has not been established. In the absence of sufficient data, the use during pregnancy and lactation is not recommended.
Side effects:
Gastrointestinal reactions (nausea, vomiting, and diarrhoea) have been reported. The frequency is not known. Allergic reactions (urticaria, skin rash, dyspnoea) have been reported. The frequency is not known.
RRP (ex-VAT):£5.20
Legal category:GSL.
PL holder:McNeil Products Limited,50 –100 Holmers Farm Way, High Wycombe, Buckinghamshire, HP12 4EG.
PL number:THR 15513/0185
Date of prep:14 April 2022
Nicorette product information
Nicorette QuickMist 1mg/spray mouthspray (nicotine),
Nicorette QuickMist Cool Berry 1mg/spray mouthspray (nicotine)
& Nicorette Quickmist SmartTrack 1mg/spray Mouthspray (nicotine)
Presentation:
Oromucosal spray. Each 0.07 ml contains 1mg nicotine, corresponding to 1mg nicotine/spray dose.
Uses:
Nicorette QuickMist relieves and/or prevents cravings and nicotine withdrawal symptoms in nicotine dependence, such as those arising from the use of tobacco or electronic cigarettes. Indicated to aid quitting or reduction prior to quitting, to assist those who are unwilling or unable to use such products, and as a safer alternative to smoking tobacco for smokers and those around them. It is indicated in pregnant and lactating women making a quit attempt.
Dosage:
Adults and Children over 12 years of age: The patient should make every effort to stop smoking or vaping completely during treatment with Nicorette QuickMist. One or two sprays used when normally would smoke or vape or if cravings emerge. Maximum dose of 4 sprays per hour, not exceeding 2 sprays per dosing episode, and 64 sprays in any 24-hour period. Nicorette QuickMist should be used whenever the urge to smoke or vape is felt or to prevent cravings in situations where these are likely to occur. Patients able to stop smoking/vaping immediately should initially replace all their cigarettes/e-cigarettes with the Nicorette QuickMist and as soon as they are able, reduce the number of sprays used until they have stopped completely. Patients aiming to reduce cigarettes/ e-cigarettes should use the Mouthspray between smoking/vaping episodes, as needed, to prolong smoke/vape-free intervals and to reduce their use as much as possible. As soon as they are ready patients should aim to quit smoking/vaping completely.
Contraindications:
Children under 12 years of age. Known hypersensitivity to nicotine or any excipients in the mouthspray.
Precautions:
Smokers: Underlying cardiovascular disease. Stopping smoking may alter the metabolism of certain drugs. Transferred dependence is rare and both less harmful and easier to break than smoking dependence. Smokers or vapers: Diabetes mellitus, G.I disease, uncontrolled hyperthyroidism, phaeochromocytoma, hepatic or renal impairment, seizures. May enhance the haemodynamic effects of, and pain response to, adenosine. Presence of a small amount of butylated hydroxytoluene (BHT) may cause local skin reactions (e.g. contact dermatitis), or irritation to the eyes and mucous membranes. Keep out of reach and sight of children. Dispose of with care. Do not spray into eyes whilst administering the spray.
Pregnancy & lactation:
Smoking cessation during pregnancy should be achieved without NRT. However, if the mother cannot (or is considered unlikely to) quit without pharmacological support, NRT may be used only after consulting a healthcare professional. There is no or limited data regarding the effect of vaping in pregnancy.
Side effects:
Very common:Headache, throat irritation, nausea, hiccups.
Common: Toothache, cough, hypersensitivity, burning sensation, dizziness, dysgeusia, paraesthesia, abdominal pain, diarrhoea, dry mouth, flatulence, salivary hypersecretion, stomatitis, vomiting, dyspepsia, fatigue.
Uncommon: Abnormal dreams, palpitations, tachycardia, flushing, hypertension, bronchospasm, dysphonia, dyspnoea, nasal congestion, sneezing, throat tightness, eructation, glossitis, oral mucosal blistering and exfoliation, paraesthesia oral, dry skin, urticaria, angioedema, hyperhidrosis, pruritus, rash, erythema, pain in jaw, asthenia, chest discomfort and pain, malaise, oropharyngeal pain, rhinorrhea, gingivitis, musculoskeletal pain, hyperhidrosis.
Rare: Dysphagia, hypoaesthesia oral, retching.
Not known: Atrial fibrillation, anaphylactic reaction, blurred vision, lacrimation increased, dry throat, GI discomfort, lip pain, muscle tightness, angioedema, erythema, seizures.
RRP (ex VAT): Nicorette QuickMist 1mg/spray mouthspray: 1 dispenser pack £21.23, 2 dispenser pack £33.61, Nicorette QuickMist Cool Berry 1mg/spray mouthspray: 1 dispenser pack £21.23, 2 dispenser pack £33.61, Nicorette Quickmist SmartTrack 1mg/spray mouthspray: 1 pack dispenser £22.41, 2 dispenser pack £36.24
Legal category: GSL
PL holder: McNeil Products Ltd, 50-100 Holmers Farm Way, High Wycombe, HP12 4EG
PL number: Nicorette QuickMist 1mg/spray mouthspray: 15513/0357; Nicorette QuickMist Cool Berry 1mg/spray mouthspray: 15513/0395; Nicorette Quickmist SmartTrack 1mg/spray mouthspray: 15513/0357
Date of preparation: 18 Nov 22